Clinical Insight to Corporate Value: The Strategic Evolution of PatchClamp
Original Publication – Design News May 1, 2025: Scott Thielman, CTO & Cofounder, Product Creation Studiohttps://www.designnews.com/medical/clinical-insight-corporate-value-the-strategic-evolution-of-patchclampThe development of PatchClamp, a novel solution for preventing postoperative leakage of cerebrospinal fluid, demonstrates the importance of engineering partnerships and intellectual property protection. The dural repair system aims to improve patient outcomes and surgical efficiency.
At a Glance
Minimally invasive procedures minimize tissue impact, yet confined spaces can limit options.
A neurosurgeon envisioned a novel way to internally patch the protective membrane surrounding the brain & spine.
Developing the solution into a marketable product involved a team of engineering, testing, & patent experts.
Article
In the depths of a minimally invasive neurosurgical procedure, Dr. Marc Mayberg faced a familiar yet frustrating challenge. Working through an opening barely wider than a finger, at a depth of nearly four inches, he needed to repair the delicate membrane protecting his patient's brain. The traditional surgical techniques—developed for open procedures with room to maneuver—became nearly impossible to execute in this confined space. Each attempt to suture or patch the tissue heightened the risk of complications that could extend his patient's recovery or lead to serious infections.
For surgeons like Dr. Mayberg, this moment represents more than a technical hurdle—it embodies the growing tension between surgical innovation and practical limitations. While minimally invasive approaches promise faster recovery and reduced trauma, they introduce complexities that traditional surgical tools weren't designed to address. In this gap between surgical advancement and surgical practice lies an opportunity for transformative innovation.
"Minimally invasive surgery of the brain and spine is increasingly performed because it's associated with less postoperative pain, reduced need for narcotics, and faster recovery," explains Dr. Mayberg, now CEO of PatchClamp MedTech. "But operating through narrow access ports creates unique challenges, particularly when repairing the dura—the protective membrane surrounding the brain and spine."
Dr. Mayberg's insight went beyond identifying a technical problem. He envisioned a fundamentally different approach: rather than struggling to manipulate surgical tools through a narrow port, what if surgeons could deploy a repair from the inside? This conceptual shift—seemingly simple yet profound in its implications—would launch a development journey that exemplifies how clinical insight, strategic development partnerships, and thoughtful execution can transform surgical possibilities.
The Human Impact Behind Technical Innovation
The stakes in neurosurgery are inherently high, but they become even more critical when dealing with cerebrospinal fluid (CSF) leakage. With minimally invasive surgical procedures growing at a remarkable 40% compound annual growth rate, the challenge of working through narrow surgical ports affects an ever-increasing number of patients. For these individuals, complications from inadequate dural repair can mean extended hospital stays, increased risk of infection, and potentially serious complications like meningitis.
"Most minimally invasive cases are performed at a depth of about 8-10cm through an access port measuring just 8-24mm in diameter," explains Dr. Mayberg. "The pressure of cerebrospinal fluid is higher than atmospheric pressure, so any opening in the dura causes persistent leakage. This isn't just a technical challenge—it's a direct threat to patient recovery."
The human cost is substantial. While major medical device companies like Medtronic and Integra offer FDA-approved bio-absorbable patches for dural repair, these solutions are designed for open surgery where surgeons have room to maneuver. When applied through minimally invasive ports, traditional approaches often fall short, leading to increased hospital readmissions, extended trauma, and heightened risk of infection.
This reality drove Dr. Mayberg's vision for a revolutionary approach: rather than struggling to suture or patch the dura from the outside through a narrow port, what if surgeons could deploy a repair from the inside? This seemingly simple conceptual shift opened the door to an entirely new paradigm in surgical repair—one that could potentially reduce post-surgery hospital stays, decrease observation time, and significantly lower the risk of complications.
"The basic premise to any novel medical technology is that the impact needs to be material and the use of the new device must lead to better outcomes at a lower cost," notes Joe Piper, board member and advisor for PatchClamp. "Our solution checks every box—reducing time in the operating room due to the simplicity of placing the patch through the same minimally invasive opening, while potentially decreasing costly hospital readmissions."
What makes this innovation particularly compelling is its potential reach beyond neurosurgery. The fundamental approach—delivering and securing repair materials from the interior of tissue membranes—could transform procedures across multiple surgical specialties, offering a new standard for minimally invasive tissue repair that prioritizes both surgical efficiency and patient recovery.
Yet transforming this clinical vision into a working reality demanded more than just recognizing the opportunity. It required bridging the delicate gap between surgical intuition and engineering possibility—a space where many promising medical innovations falter. For the development team, this meant not only solving complex technical challenges but understanding the subtle choreography of surgical procedures at an intimate level. As they moved from concept to creation, each development decision needed to balance multiple competing demands: surgical precision, ease of use, regulatory requirements, and above all, patient safety.
Translating Vision into Reality: The Innovation in Action
While the vision of interior tissue repair offered compelling possibilities, translating this concept into a working surgical device demanded both technical capability and deep understanding of surgical workflow. At its core, PatchClamp's breakthrough lies in its elegant reversal of traditional surgical repair approaches. Rather than suture a patch to the outside of the dura, the system delivers a bio-absorbable patch through the same minimally invasive opening used for the primary procedure. This patch is deployed on the inner surface of the dura—where cerebrospinal fluid pressure actually helps maintain its position—and secured by a specially designed clasp on the outer surface.
Figure 1 - A bioresorbable PatchClamp prototype fabricated at PCS and used for preclinical testing. The circular patch is deployed on the inside of the dura while the clasp secures it in place.
The innovation's elegance masks considerable technical sophistication. Through a low-profile, single-hand applicator about the width of a drinking straw, surgeons can deliver and secure repairs even in the most challenging surgical approaches. The device's design coordinates multiple precision mechanisms: a system for protecting and then deploying the patch material, a method for precisely positioning the outer clasp, and controls that allow surgeons to adjust or remove the repair if needed—all while working at depth through a minimally invasive port.
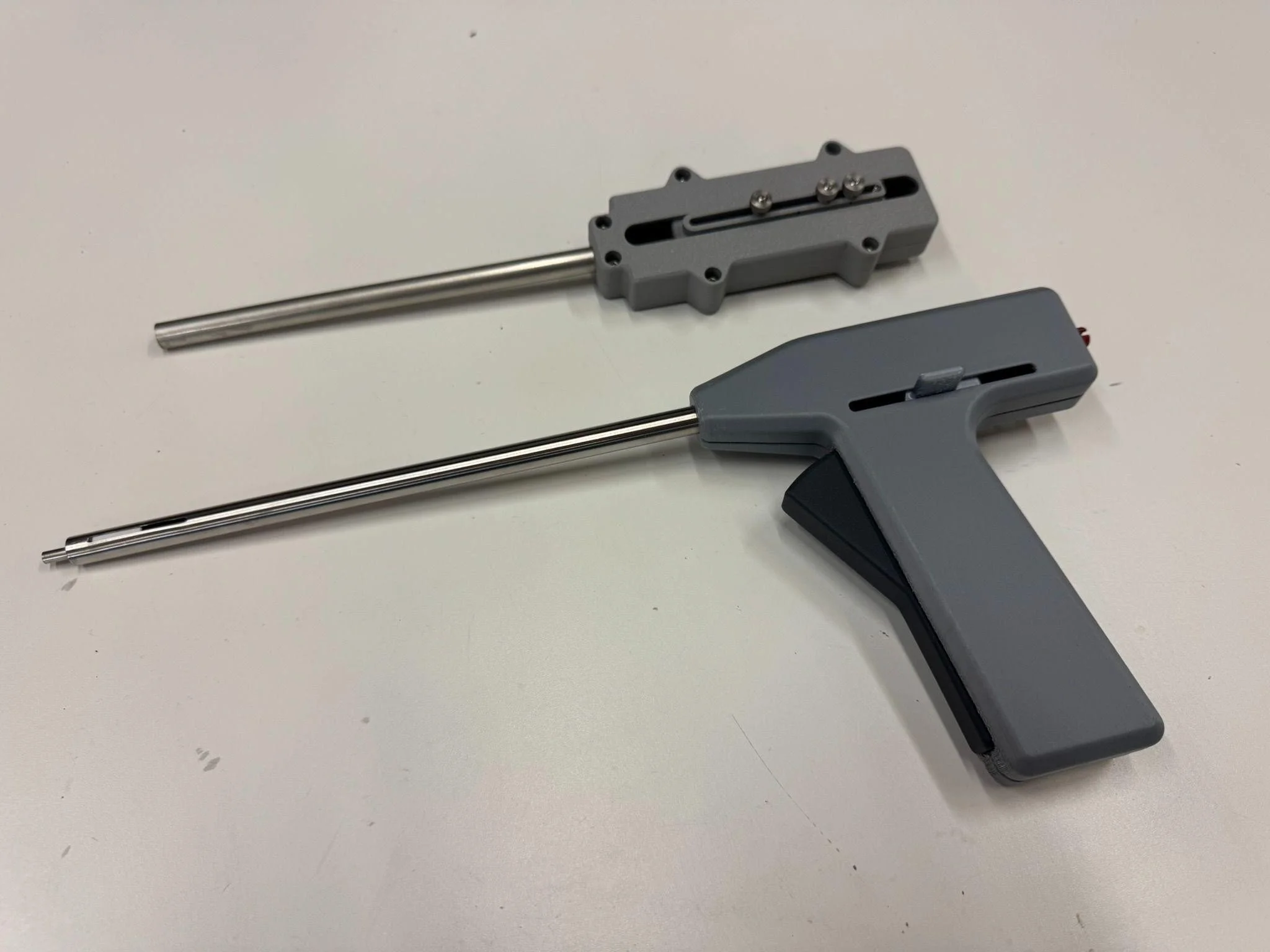
"One of our biggest challenges was creating a deployment sequence that felt natural and confident in the surgeon's hand," explains Adam Smith, Senior Mechanical Engineer at PCS. "Using a teeter-totter style trigger allowed precise control in both directions, while our sequencing mechanism coordinated springs, rack and pinion, and linkages to deploy the patch, position the clasp, and release the assembly—all through intuitive single-handed control."
Figure 2 - The evolution of the deployment handle as shown by the early test unit (top) and the more recent unit with the teeter-totter style handle (bottom).
This attention to surgical workflow exemplifies how breakthrough medical innovations must bridge the gap between technical capability and clinical usability. The team's focus wasn't just on solving the mechanical challenge of patch deployment; it was on creating a solution that would integrate seamlessly into existing surgical procedures while delivering immediate, verifiable results.
The Art of Technical Problem-Solving
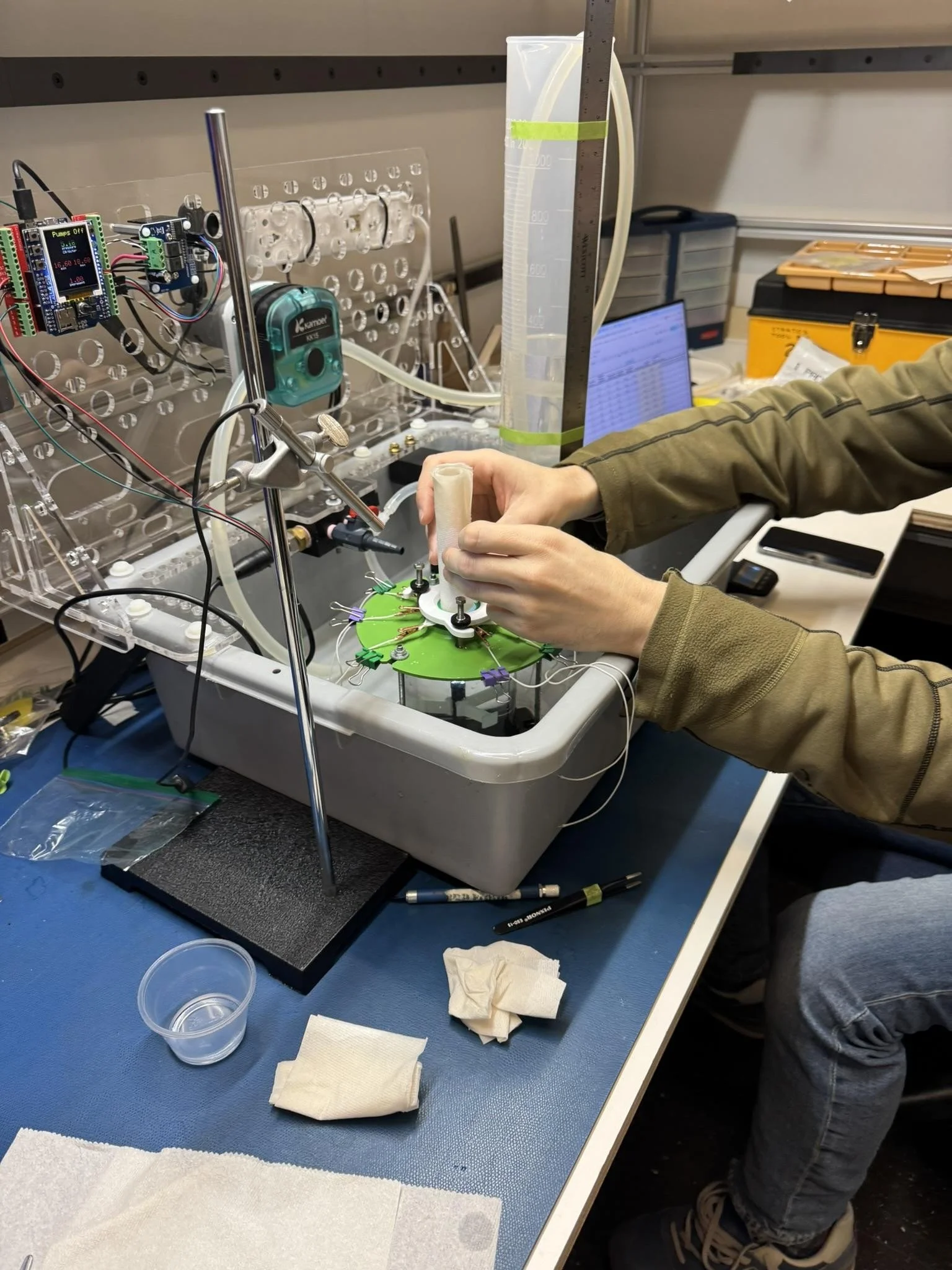
The challenge of validating an entirely new approach to tissue repair demanded more than conventional bench testing. In a corner of the PCS lab, Adam Smith and David Desmarais created a testing system that elegantly addressed a fundamental surgical reality: cerebrospinal fluid pressure.
"During surgery, spinal fluid pressures are typically in the range of 0 to 35 centimeters of water column, with a normal range being between 7 and 18 centimeters," explains Smith. Their solution combined simplicity with precision—a tall beaker and peristaltic pump connected to a pressure sensor that could maintain precise fluid levels automatically. "The pump would maintain the water column elevation using the pressure sensor as a reference," Smith notes, enabling the team to replicate and monitor the exact pressures surgeons encounter during procedures.
The test setup's evolution reflected the team's commitment to clinical authenticity. Early iterations included an elaborate pre-tensioning fixture for bovine dura, but Dr. Mayberg's insights led them to refine their approach. "Marc was our primary source for validating the test conditions," Desmarais explains. "He qualitatively assessed if the tissues were representative of clinical conditions."
This close collaboration between engineering precision and clinical insight led to unexpected breakthroughs. "The first finding was that the original concept of using a spring system to force the patch into the open position when deployed faced geometry and material limitations that made it nearly impossible," Desmarais recalls. "The second finding was that forcing the patch open wasn't necessary with a subset of the patch materials. A couple of them had enough stiffness to pop open into a disc when released, and so we abandoned the concept of an inner spring system."
The test system's modular design proved crucial, allowing quick modifications as new insights emerged. While Desmarais modestly notes that "the test setup was fairly conventional and there's nothing particularly innovative about it," its real value lay in how it enabled rapid learning and validation. The integration of pressure monitoring, visual feedback through clear acrylic chambers, and precise control over test conditions created an environment where clinical vision could meet engineering reality.
"The insights gained from experiments performed at PCS will enable us to proceed with pre-clinical proof-of-concept studies," reflects Dr. Mayberg. "We were able to demonstrate the consistent ability of PatchClamp deployed from a 10mm applicator to provide a watertight seal at CSF pressures at and above those seen in human clinical situations."
Figure 3 - Dr. Mayberg at work at the PCS lab.
Figure 4 - PCS engineer, David Desmarais, measures leakage rate during evaluation testing.
Figure 5 - A tray of printed and machined parts to support concept refinement and testing of the patch assembly.
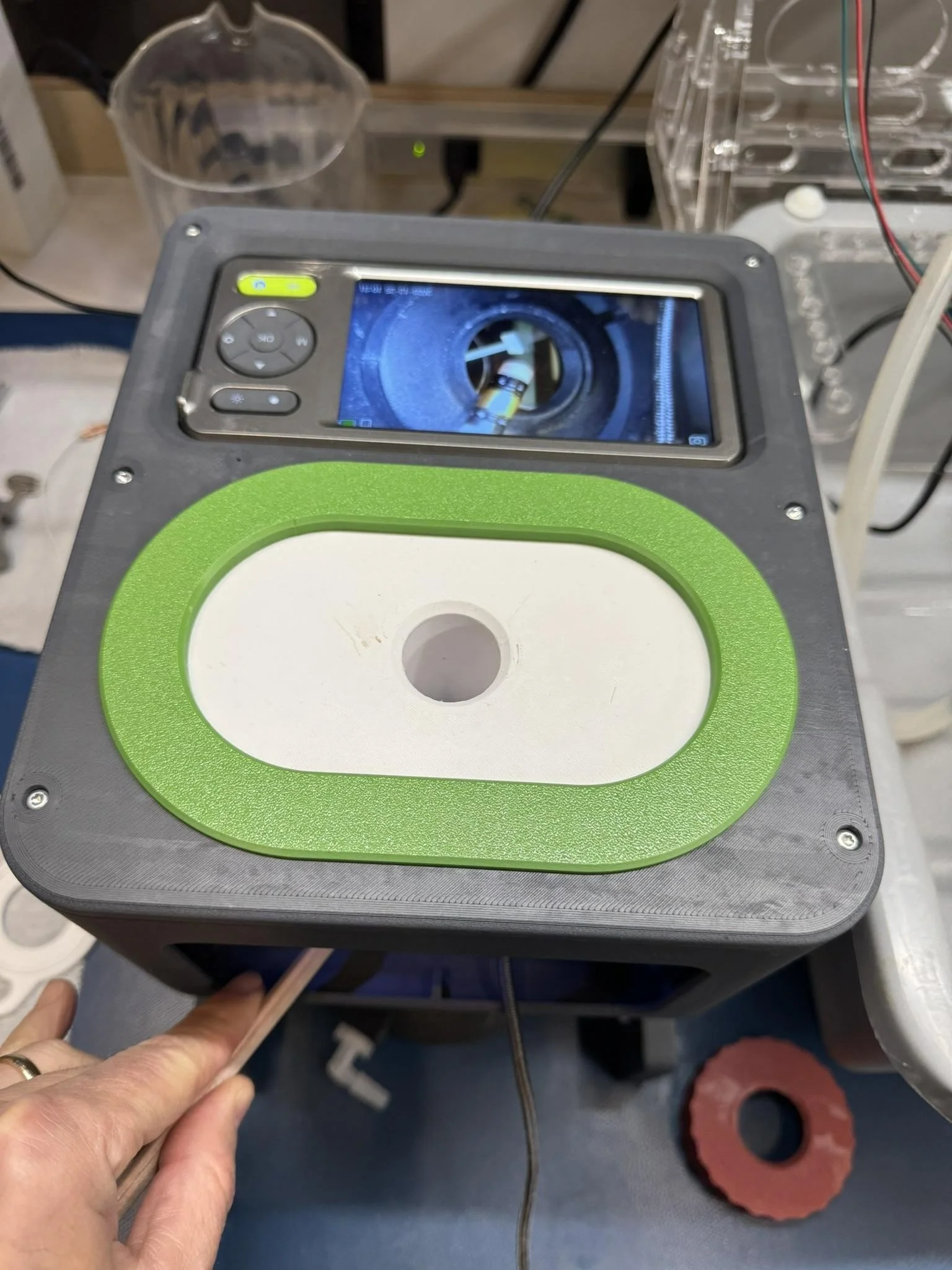
Figure 6 - An innovative camera box for simulated patch placement with the bench setup.
This holistic approach to technical validation—combining rigorous engineering with deep clinical insight—exemplifies how successful medical device development transcends pure technical problem-solving. The PatchClamp journey reveals a fundamental truth: transformative innovation emerges when technical excellence aligns with human understanding. It's not enough to create solutions that work in theory; they must resonate with the realities of surgical practice while meeting the exacting standards of medical device regulation.
From Clinical Innovation to Market Impact
This intimate understanding of surgical challenges resonates strongly with corporate venture teams seeking transformative technologies. Joe Piper, board member and advisor for PatchClamp, sees particular strategic value in how the innovation complements existing market solutions: "Most major MedTech companies have an FDA-approved bio-absorbable patch designed to be glued or stitched to the exterior of the dura post MIS procedure," he notes. "The device will reduce post-surgery hospital stays, be more effective than stitching or exterior patches, reducing the risk of infection and expensive hospital re-admissions, and time in the OR will be reduced due to the simplicity of placing the patch through the same MIS opening."
The team's approach to regulatory strategy reflects this market understanding. By pursuing an abbreviated 510(k) approval pathway using FDA-approved patch materials, they've created an efficient path to market that resonates with potential corporate partners. As Piper explains, "MedTech companies that supply neurosurgeons FDA-approved patch material will vie to license PatchClamp’s technology to differentiate their products on the market and make them more safe and effective."
But perhaps most compelling is the technology's broader potential. "PatchClamp patents allow for its use in repairing openings using MIS in any tissue membrane," Dr. Mayberg explains, "including gastrointestinal, genitourinary, pulmonary, and ENT applications." This extensibility hasn't gone unnoticed by Gary Myles, IP Counsel for PatchClamp, who has structured their patent portfolio strategically: "The original patents broadly cover the general aspects of the device present in both first and second-generation designs," he notes. "Patents focused on the second-generation devices will complement the original patent protection and extend the term by several years."
Protecting Innovation While Embracing Discovery
The moments of technical discovery emerging from the PCS lab didn't just refine the device's design—they opened new possibilities for protecting and extending the innovation's value. For Gary Myles, each breakthrough represented an opportunity to strengthen the technology's strategic position.
As the team refined their approach, Myles witnessed how testing revealed possibilities beyond mere technical improvement. "These insights opened entirely new avenues for innovation," he explains. "One particularly powerful discovery emerged when the team found that retaining the clasp in an opposite orientation overcame the challenge of developing suitable force necessary to retain the graft on an inner tissue surface. This wasn't just a technical solution; it was a novel approach that wasn't contemplated in our initial patent framework."
This discovery exemplifies the delicate balance in protecting medical device innovations. The initial patents needed to be broad enough to cover the core concept while leaving room for the inevitable evolution that comes through development. Myles and his team responded with what he calls a "layered strategy"—the original patents broadly protect the fundamental innovation, while subsequent filings cover the refined approaches that emerged through development.
To accelerate this protection, the team took an aggressive early approach, using the USPTO's Track 1 program to secure their first patent in just seven months. This early success created a foundation for global protection, leading to quick approvals in key markets like Japan. "Since our objective is to partner with one of the major medical device companies," Myles notes, "we focus our patent filings in those countries most important to target companies."
But perhaps most strategically valuable was the team's approach to keeping certain development pathways open for future partners. By maintaining pending patent applications covering second-generation innovations, they've created additional value for potential corporate collaborators who might see opportunities in markets PatchClamp hasn't yet considered.
Orchestrating Innovation: The Art of Resource Management
Behind every breakthrough medical device lies not just technical innovation, but the careful orchestration of resources, relationships, and competing priorities. For the PatchClamp project, this delicate balance fell to Gina Longman, Senior Project Manager at PCS, who faced the classic challenge of early-stage medical device development: how to maximize learning and progress while carefully managing limited resources.
Figure 7 - The PatchClamp experimental lab space at PCS. Shown in image: Scott Thielman, (left) CTO of PCS; Gina Longman, PCS Sr. Project Manager; David Desmarais (next to equipment), PCS Sr. Mechanical Engineer; Joe Piper (seated with smartphone), PatchClamp Board Member; Dr. Marc Mayberg, PatchClamp Founder; not shown: Adam Smith, PCS Sr. Mechanical Engineer, Gary Myles, PatchClamp Counsel; Hans Lundin, PatchClamp Board Member.
"Early-stage medical device development often feels like solving a puzzle where every piece affects all the others," Longman explains. "You need data to secure funding, but you need funding to generate data. Success comes from structuring the development program to systematically address critical questions while maintaining flexibility to adapt as we learn."
The approach Longman and her team developed exemplifies the art of strategic project management in innovation. They created a carefully staged development program broken into distinct phases - Intake, Development, and Refinement - each with clear objectives and deliverables. This structured yet flexible approach allowed the team to:
● Generate compelling evidence of feasibility through early mockups and testing
● Maintain focused development priorities despite limited resources
● Create clear decision points for advancing the technology
● Enable efficient collaboration between clinical and technical team members
But perhaps more crucial than technical planning was Longman's focus on fostering effective communication between stakeholders. By establishing structured weekly status meetings while maintaining flexible channels for technical discussions, the team could maintain alignment between immediate development tasks and longer-term goals.
"In early-stage development, you're not just managing tasks and timelines," Longman notes. "You're creating an environment where innovation can flourish while maintaining the focus and discipline needed to reach critical milestones. This means being very clear about assumptions, responsibilities, and constraints from the beginning."
This principle is reflected in how detailed project assumptions were documented upfront - from the scope of prototype development to the handling of bio-materials - ensuring all team members understood both the possibilities and limitations of the current phase. Similarly, clear delineation of client and PCS responsibilities helped prevent misalignments that could derail progress.
For other program managers facing similar challenges in early-stage medical device development, the PatchClamp experience offers valuable insights about structuring development programs to maximize learning and progress with limited resources. It demonstrates how thoughtful program management can help bridge the gap between clinical vision and technical execution, creating an environment where breakthrough innovation becomes possible.
Lessons from the PatchClamp Journey
Behind every breakthrough medical device lies a story of human insight, persistence, and collaborative spirit. The PatchClamp journey illustrates how transformative innovation emerges not just from technical excellence, but from a deep understanding of the human experience—both in the operating room and throughout the development process. It's a narrative that offers valuable insights for organizations seeking to nurture breakthrough innovations in complex regulatory environments.
The story began with Dr. Mayberg's intimate understanding of surgical challenges, but it was the team's ability to translate this clinical insight into meaningful innovation that proved transformative. "Every development decision was made with an eye toward creating value not just for patients and clinicians," reflects Dr. Mayberg, "but for the entire ecosystem of stakeholders who could help scale this technology's impact." This holistic perspective—considering both immediate surgical needs and broader strategic implications—created a foundation for sustainable innovation.
The project's success hinged on more than just technical problem-solving. It required orchestrating a delicate balance of resources, relationships, and competing priorities. Gina Longman, Senior Project Manager at PCS, found herself navigating what she calls "the innovation paradox"—the need to generate compelling evidence while carefully managing limited resources. "Early-stage medical device development often feels like solving a puzzle where every piece affects all the others," she explains. "Success comes from structuring the development program to systematically address critical questions while maintaining flexibility to adapt as we learn."
This adaptive approach proved crucial as the team moved from concept to reality. The engineering team's willingness to challenge assumptions and embrace unexpected discoveries led to elegant solutions that might have been overlooked with a more rigid development process. When David Desmarais and his colleagues discovered they could eliminate the inner spring system by leveraging material properties, it exemplified how technical excellence combined with creative thinking can yield simpler, more effective solutions.
But perhaps most significantly, the PatchClamp story demonstrates how breakthrough innovation requires more than just solving technical problems—it demands creating solutions that resonate deeply with human needs while building value for all stakeholders. The team's approach to intellectual property protection, led by Gary Myles, exemplifies this comprehensive thinking: "We structured our patent portfolio to protect both current applications and future developments," he notes, "creating multiple opportunities for potential corporate partners to extend their own IP positions in key markets."
Looking ahead, the implications of this work extend far beyond neurosurgery. Dr. Mayberg envisions applications across multiple surgical specialties, from gastrointestinal procedures to pulmonary interventions. But more than specific applications, the PatchClamp journey offers a blueprint for nurturing medical innovation in today's complex healthcare landscape. It reminds us that behind every successful device lies a human story—one driven by the persistent vision of clinicians, engineers, and development partners working together to improve patient care.
The key lessons resonate across industries:
● Begin with deep human understanding rather than pure technical possibility
● Choose strategic partners who share your commitment to meaningful innovation
● Build value through careful validation that considers all stakeholders
● Protect innovation with an eye toward future possibilities and partnerships
But perhaps the most profound lesson is that transformative innovation emerges from the delicate interplay between clinical insight, strategic thinking, and focused execution. When these elements align, breakthrough solutions become not just possible but inevitable.